While both AL Amyloidosis and Multiple Myeloma are plasma cell disorders and share similar patient care treatments, they diverge in pathogenic mechanisms. In this presentation, Dr. Giada Bianchi from Brigham & Women’s Amyloidosis Program shares that 10-15% of Multiple Myeloma patients will experience overlapping AL Amyloidosis, elevating the importance of understanding the similarities and differences in diagnostic criteria and patient care.
Slider
Patient Insights: I have to advocate for myself
With a rare disease, it is difficult to expect the healthcare community to be fully knowledgeable. Thus being a self-advocate is critically important. Our patient speakers at the Amyloidosis Speakers Bureau are powerful educators and offer compelling insights. Have a listen to this brief clip from Dan.
Inflation Reduction Act of 2022 & Amyloidosis
What does the Inflation Reduction Act of 2022 mean for the rare disease community and Amyloidosis patients?
Those of us in the amyloidosis patient community hear repeatedly that the key to better outcomes is early diagnosis and treatment. Much progress continues to be made in raising awareness in the medical community about these rare diseases. The goal is to help physicians consider and test for the diseases but there remains more to be done. For many reasons, inconsistent and widely varied multi system symptoms, misdiagnosis based on symptoms that mimic more typical conditions, lack of familiarity with the disease and more, amyloidosis remains widely underdiagnosed. Speaker Bureaus with patients telling their stories to medical students and residents, patient support groups that invite physicians to explain their experience in diagnosing amyloidosis, and a variety of medical education opportunities continue to help raise awareness. Once a patient does receive a correct diagnosis and is referred for treatment another journey begins. The currently available and most effective therapies are almost uniformly far too costly for patients to bear. Copay assistance programs from pharmaceutical companies provide much needed help but many Medicare patients are forced to “compete” for grant monies and in some cases still have exorbitant out of pocket copays. Additionally, it is often a lengthy and difficult process to obtain insurance approval for coverage of these costly medications with prescribers and patients spending much time, years in some cases, working through the denials and appeals and still not always receiving approval.
Unlike twenty years ago there are now several good FDA-approved treatment options for amyloidosis in the U.S. These slow the progression of the debilitating symptoms of the diseases, but none cure or reverse the damage already done. Considerable research and development continues in a search for more effective treatments with the goal of finding a cure, but the search is costly and time consuming.
Until the early 1980s pharmaceutical companies had no financial incentive to spend the enormous amounts of money required to develop a new drug to treat the small populations of patients with a rare disease, defined as “affecting fewer than 200,000 US citizens.”1 The market for the resulting drugs would not be large enough to make it financially feasible. The Orphan Drug Act (ODA) of 19832 was created to help with exactly this sort of situation. Prior to its adoption, “only ten products for the treatment of rare disease were approved for use in the U.S.”1 This is a stunning fact when one considers that according to the Genetic and Rare diseases Information Center of the National Institutes of Health, known rare diseases now number more than six to ten thousand and afflict more than thirty million people.3
“In the early 1980s, families, advocates, and leaders of several rare disease patient organizations formed an ad hoc coalition to focus attention on this problem. That coalition was instrumental in passage of the Orphan Drug Act, a landmark bill that created financial incentives for the development of treatments for rare diseases…In 1983, that coalition became the National Organization for Rare Disorders, or NORD…” 4
Pressure from this coalition, the medical community, and the public resulted in the passage of the Orphan Drug Act of 1983 (ODA).
“…Since the passage of the Orphan Drug Act of 1983, the US Food and Drug Administration (FDA) has approved more than 500 orphan products and rare disease therapies, and orphan drugs currently make up more than 50% of new drug approvals at FDA.” 4
The Act was also intended to address what was perceived by many in the medical community as a lag of available and appropriately effective therapies in the US compared with the rest of the world. This was usually attributed to the tightening of drug safety laws required by the Kefauver-Harris Bill of 1962 which amended the Food, Drug, and Cosmetic Act enacted after the horrible events with thalidomide in years just prior. This amendment required “…all drugs to be proven safe and effective by adequate well-controlled studies before being approved for the U.S. market. While this improved public protection from potentially dangerous pharmaceuticals, it also dramatically increased the costs associated with drug development. Consequently, pharmaceutical companies began to focus on developing treatments for common diseases with large potential markets in order to maximize the possibility of recouping research and development costs and generating significant revenues.1
The ODA with its provisions for seven-year market exclusivity for orphan drugs, generous tax credits, drug development grants, expanded access to approved orphan drugs, and FDA fee reductions was intended to correct the unintended consequences of the Kefauver-Harris Bill and did result in a dramatic increase in new medications for rare diseases. The ODA was widely hailed as a great success5 but an unintended consequence of the incentives was its contribution to the escalating price of prescriptions.
By the late 1980s the concern for the rare diseases had shifted from the need for incentives to develop them to the unprecedented profits pharmaceutical companies were making from these orphan drugs and the ever escalating prices and the inability of patients to afford them. An excellent history of the changing landscape and legislation around treatments for rare diseases can be found in the National Institutes of Health National Library of Medicine’s Orphans in the Market: the History of Orphan Drug Policy.6
In response to public outcry, the Inflation Reduction Act of 2022 (IRA) which, among its many other provisions, included new laws to address prescription costs especially for elder Americans with Medicare coverage. The law, Public Law No: 117-169, was a response to the Comprehensive Plan for Addressing High Drug Prices: A Report to the Executive Order on Competition in the American Economy dated September 9, 2021.7 The text of the act can be read in Section B – Prescription Drug Pricing Reform which discusses how price lowering is intended to occur. One provision of the law allows Health and Human Services to negotiate with pharmaceutical companies the “maximum fair price” for a limited list of the most commonly used drugs for patients with Medicare coverage. For a detailed review of the provisions of this law as it relates to Medicare drug coverage please see Explaining the Prescription Drug Provisions in the Inflation Reduction Act, January 24, 2023 8 or Fact Sheet: Medicare Prescription Drug Inflation Rebate Program Part B Rebatable Drug Coinsurance Reduction, March 2023.9 Transition to the new policies are beginning now and will continue to roll out over the next few years. Initial reaction to the law has been positive but with more understanding about how the law works some of this enthusiasm has shifted to concern about the unintended consequences especially for the rare disease community.
A law to help reduce the cost of drugs should be a cause for celebration and much of the IRA appears to offer just that, but in recent months several opinion pieces and responses to the legislation have suggested unintended negative consequences. A balanced consideration from the National Organization of Rare Disorders (NORD) looks at the pros and cons of the IRA10 and a letter to then Majority Leader Schumer August 5, 2022 from the Everyday Foundation for Rare Diseases, another respected non-profit advocacy group for the rare disease community, strongly advocates for a reconsideration of the language in the act limiting the exclusions of rare disease drugs to “…those for only one disease and one approved indication…” 11 In March of this year an article from the Council for Affordable Health Coverage entitled How the Inflation Reduction Act is Impacting Rare Disease Patients12 published a blog that explored the impact of the IRA with a focus on these consequences. Of particular interest is the focus on research and development. The law has already been cited by Alnylam and Eli Lilly as the reasons for stopping research on certain secondary uses for specific drugs. As recently as March and July of 2023, two lawsuits have been initiated to challenge provisions of the IRA, one by Merck and the other by the U.S. Chamber of Commerce. Each argues that the price negotiating processes outlined in the law will work to “jeopardize medical breakthroughs for individuals with life-threatening and chronic illnesses.”13
Another interesting exploration of the potential downsides of the current law, Inflation Reduction Act’s Unintended consequences, can be found in recent postings from PhRMA.Org, a trade group which lobbies on behalf of the U.S. pharmaceutical industry14. While the perspective is from that of the industry, the arguments made are reflected in both recent lawsuits and in the stopping of research and development for secondary applications of drugs previously approved for single disease treatment. Consider information about Alnylam’s decision to curtail a planned phase 3 study of the drug Amvuttra, currently FDA approved for the treatment of hATTR-PN hereditary amyloidosis polyneuropathy, that was also showing potential for use in the treatment of Stargardt, a rare eye disease.15
Alnylam attributed the pause to Biden’s Inflation Reduction Act, which allows Medicare to directly negotiate prices of some high-expenditure drugs. Drugs with one single orphan drug designation is exempt from potential price negotiations, a term that may discourage companies from exploring approvals in additional indications, Alnylam CEO Yvonne Greenstreet noted on the call. Because Amvuttra and Onpattro already has an orphan status in ATTR, an additional orphan label could theoretically open it for potential pricing scrutiny. 16,17
SUMMARY
As awareness about amyloidosis continues to increase and more and more patients are properly diagnosed and hoping to begin therapy to help mitigate the disabling and too often fatal progress of the diseases, it is important to consider both the financial burden to the patient and the availability of new and better drugs to treat or even cure the disease. Despite the well-intended goal of lowering prescription drug costs generally and allowing drug prices for Medicare recipients to be negotiated, there remains much to unravel about the effects of the new Inflation Reduction Act. It will likely save many people money but there is concern, especially regarding the medications for the treatment of rare diseases such as amyloidosis, and about what appears to be a disincentive to pharmaceutical companies to engage in costly research and development to treat these rare diseases. The challenge to diagnosis and providing effective, affordable treatment to amyloidosis patients continues.
CITATIONS
1https://www.researchgate.net/publication/7239721_Orphan_drug_policies_implications_for_the_U nited_States_Canada_and_developing_countries, Cheung, Cohen & Illingworth download
2https://www.govinfo.gov/content/pkg/STATUTE-96/pdf/STATUTE-96-Pg2049.pdf
3https://rarediseases.info.nih.gov/
4https://rarediseases.org/about-us/history/
5https://oig.hhs.gov/oei/reports/oei-09-00-00380.pdf
6https://pubmed.ncbi.nlm.nih.gov/31384102/
7https://www.congress.gov/117/plaws/publ169/PLAW-117publ169.pdf
9https://www.cms.gov/files/document/fact-sheet-part-b-rebatable-drug-coinsurance-reduction.pdf
13https://www.advisory.com/daily-briefing/2023/06/14/ira-lawsuits
14https://phrma.org/inflation-reduction-act
Note: Fierce Pharma is a news publication of happenings in the Pharmaceutical industry
17https://rapport.bio/all-stories/alnylam-is-doing-what-the-ira-is-telling-it-to-do
ADDITIONAL READING
ORPHAN DRUGS AND RARE DISEASES
https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/CFRSearch.cfm?fr=316.20https://www.fda.gov/patients/rare-diseases-fda
INFLATION REDUCTION ACT
https://www.cms.gov/files/document/fact-sheet-part-b-rebatable-drug-coinsurance-reduction.pdf
Multidisciplinary Care for Cardiac Amyloidosis Patients

Multi-systemic diseases such as amyloidosis are complex to diagnose, but also complex in treatment and ongoing patient care. It takes a village. In this seminal piece, the American College of Cardiology (ACC) provides an Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis.
According to Dr. Vaishali Sanchorawala, Director of the Amyloidosis Center at Boston Medical Center, “The results and progress in the therapeutic landscape of systemic amyloidosis are unbelievable, unprecedented and unheard of for this uniformly fatal disease of the 1990s. But they are not enough, and therefore we need to work together to make a difference.”
This paper is an absolute must-read for cardiologists and other specialties such as neurology, gastroenterology, nephrology and hematology.
To read, CLICK HERE.

Thank you.
————————————————————
Source:
Kittleson M, Ruberg F, et al. 2023 ACC Expert Consensus Decision Pathway on Comprehensive Multidisciplinary Care for the Patient With Cardiac Amyloidosis. J Am Coll Cardiol. 2023 Mar, 81 (11) 1076–1126.
https://www.jacc.org/doi/10.1016/j.jacc.2022.11.022
Rebuilding the Immune System

Losing one’s immune system is a serious consequence of a stem cell transplant, putting the body at risk for viral, fungal, and bacterial infections. Rebuilding the body’s immune system is a key part of the recovery.
LOSING ONE’S IMMUNE SYSTEM FROM A STEM CELL TRANSPLANT
A stem cell transplant (SCT), whether autologous or allogeneic, begins with chemotherapy, which is intended to eradicate problematic cells. Especially when administered in high doses, chemotherapy damages the bone marrow, leaving it unable to produce enough red blood cells, white blood cells, and platelets for a period of time – hence the reason for the re-infusion (or transplant) of stem cells. Following the transplant, the body begins to re-establish its cell production.
This killing of white blood cells severely compromises the body’s immune system, making the body vulnerable to even the tamest of infections and diseases for a period of time. And while infection remains the leading cause of post-transplant complications, protection against vaccine-preventable infections also a priority. Rebuilding the immune system is, therefore, paramount to recovery.
REBUILDING THE IMMUNE SYSTEM
Rebuilding the body’s immune system happens in two ways:
1) Immune Reconstitution. After the transplant, the body begins to naturally rebuild its immune system. White blood cells typically take between 21 to 28 days to return to a near-normal level, with B- and T-cell recovery taking approximately one to three months post-transplant. Healthcare providers will closely monitor this, as the trajectory of recovery can vary from patient to patient. For example, according to the Fred Hutchinson Cancer Research Center, after chemotherapy, the immune system recovery can be slower than believed, in some cases upwards of nine months, particularly in smokers.
2) Revaccination. Vaccination is an important process used to prevent many infections and reinstate, prolong, and/or extend immunity. The process typically begins one-year post-SCT and spans approximately twelve months.
CARE DURING THE REBUILDING PERIOD
During the recovery process, it is important that patients and those in frequent contact are more cautious. Some things to consider include:
- Get minor symptoms checked out, as they could turn into something more serious
- Get revaccinated
- Avoid disease “hot spots”
- Stay active and eat well
- If a smoker, try to quit
REVACCINATION PROCESS
The topic of revaccinating SCT patients isn’t entirely straightforward. Repeat vaccinations or boosters are often crucial in reinstating, prolonging, and/or extending immunity. Patients can be tested beforehand to find out which vaccines are needed and which ones are not required due to adequate antibody levels in the blood. This is determined through a simple blood test known as a titer. Vaccines would then only be given if the titers show a lowered or absent level of protective antibodies for the disease.
Distinguishing live virus vaccines from inactivated vaccines is taken into consideration when planning the revaccination process. Patients who are immunosuppressed, including post-transplant patients, should wait at least 24 months post-SCT and until they are no longer receiving immunosuppression, free from graft-versus-host disease (GVHD), and have immunologic response before receiving live vaccines. Live virus vaccines use the weakened (attenuated) form of the virus. Inactivated vaccines are made from the killed version of the germ that causes a disease. Live virus vaccines are used to protect against:
- Measles, mumps, rubella (MMR combined vaccine)
- Live attenuated influenza vaccine
- Oral (Sabine) polio
- Rotavirus
- Smallpox
- Yellow fever
- Typhoid
- Chickenpox/Shingles (Varicella)
The typical timeline begins one-year post-SCT and extends for 12 months. It may be adjusted by healthcare professionals due to patient-specific factors such as pregnancy or active graft-versus-host disease (GVHD). Vaccinations are typically given at the following timepoints:
- 12 months post-SCT
- 14 months post-SCT
- 16 months post-SCT
- 24 months post-SCT
It is important to be aware that SCT recipients may remain immunocompromised far beyond two years post-transplant, especially individuals with chronic GVHD. Therefore, transplant patients should have their titers monitored and be appropriately revaccinated until they regain immune competence.
“HOT SPOTS” TO AVOID
Time is required to rebuild an immune system – on average two years post-SCT. This necessitates that patients be mindful of what they could be exposed to in regions that they are considering visiting. “Hot spots,” or locations which exhibit an above-normal level of disease existence, are particularly problematic to those with a compromised immune system. According to the Center for Disease Control (CDC), many developing and emerging countries demonstrate a notable level of outbreak of diseases including yellow fever, typhoid, mumps, and measles. Having said that, diseases can transcend boundaries due in part to the ease of global mobility. Per the CDC, several northeastern states in the U.S. have a high level of reported mumps cases, and mumps remains a common disease in areas such as Europe, Asia, the Pacific, and Africa. Interestingly, according to a recent study published by the Public Library of Science, a number of American states and metropolitan areas are vulnerable to become a “hot spot” with an outbreak of a vaccine-preventable disease from children whose parents opted out of vaccination. Being aware of such “hot spots,” both domestically and abroad, is important.
CONCLUDING THOUGHTS
Healthcare professionals skilled in administering different types of chemotherapies and stem cell transplants are key to monitoring and guiding patients through the rebuilding of their immune system. They will provide details with regards to the timeline and process for revaccination, ensuring patient-specific considerations are incorporated. It is important, though, for patients to understand the rebuilding of their immune system is a process that typically spans two years, during which they should maintain an appropriate level of awareness and caution.
Sources: Journal of the Advanced Practitioner in Oncology, Fred Hutchinson Cancer Research Center, Be The Match, MD Anderson Cancer Center, CNN, CIBMTR, American Society of Hematology, Center for Disease Control, Public Library of Science (PLOS).
Transplant: Inpatient vs Outpatient

There is no cure for Amyloidosis.
There are, however, an increasing number of treatment alternatives that can significantly reduce, if not eliminate, the disease and put the patient into remission. The most aggressive treatment is a stem cell transplant (SCT); sometimes referred to as a bone marrow transplant.
Stem cells are cells in the bone marrow from which all blood cells develop. This treatment aims to eradicate, typically through high-dose chemotherapy (e.g., melphalan), the faulty plasma cells which make the amyloid light chains. Once eradicated, fresh cells, harvested from the patient themselves (autologous), a donor (allogeneic), or an identical twin (syngeneic), are infused into the patient. This will help to recreate a healthy bone marrow and hopefully stop further production of the amyloid protein.
This complex treatment typically takes four to six weeks and is performed on an inpatient, outpatient, or some combination, depending on the hospital. There are meaningful differences that are important to know and incorporate into each patient’s personal situation in order to make an informed decision.
From the Healthcare Perspective
Across the country, there are multiple hospitals that perform SCTs to treat amyloidosis. While hard data is elusive, the tally of transplants at each facility, we know, is not spread evenly. We do know that Mayo Clinic (Mayo) and Boston University (BU) dominate the list and perform the majority of transplants. It may not be a surprise, then, that these two hospitals are considered amyloidosis Centers of Excellence in the U.S. They see a high volume of cases, have extensive depth and breadth of expertise, and have sophisticated diagnostic equipment. They are also the two hospitals who have pioneered performing outpatient transplants. The good news is this is evolving, with more centers across the country expanding their transplant program to treat amyloidosis.
Everyone would agree that hospitals are germ and bacteria magnets, which can be dangerous for transplant patients with low to no immune systems. BU and Mayo, for example, found patients were better able to withstand the everyday germs outside of the hospital better than the more potent ones within hospitals. This provides a strong incentive for hospitals to consider outpatient, or if they choose the inpatient route, must be ever super mindful of this reality.
There are risks with SCT, and patient safety is key. Having a patient in-house during the treatment affords the hospital maximum control during the process, while being outpatient transfers some responsibility to the caregiver, such as monitoring the patient’s temperature, food, and fluid intake. Being inpatient also affords the quickest access to experts, equipment, and drugs in the event things go awry, which does happen. Mayo has found that a meaningful percentage (38% according to Dr. Morie Gertz) of patients never need hospitalization during the SCT process; however, on the occasions where it is necessary the duration averages a handful of days.
Treating patients on an outpatient basis requires hospitals to alter their process and training, and rely on the patient and caregiver to assume a more engaged role. Without question, hospitals benefit significantly from the experience of performing high volumes of outpatient transplants. Mayo, according to Dr. Morie Gertz, performed their first SCT in March 1996, and their first outpatient SCT in September 1998. In total, they have performed 744 SCTs and currently average about 33 transplants per year. According to Dr. Vaishali Sanchorawala, BU performed their first SCT in July 1994, and their first outpatient SCT in October 1996. In total, they have performed roughly 675 SCTs for AL Amyloidosis, with an annual run rate ranging between 25 and 50. Together, these institutions have over two decades of valuable experience. According to experts, small volume and the resultant lack of experience is likely the key driver behind why hospitals elect to perform SCTs on an inpatient basis.
From the Caregiver Perspective
Caregivers play a critical role in the SCT process, working closely with the healthcare team to ensure the patient is progressing appropriately. They are so critical, in fact, that regardless of inpatient or outpatient, hospitals will not proceed with a SCT unless they are confident the patient has capable and continuous caregiver support.
The role of a caregiver varies greatly between an inpatient and outpatient process. When inpatient, the caregiver provides important emotional support, as being confined to a hospital for weeks on end can be draining and discouraging. This can range from just being present, to chatting, to light activities. Caregivers also assist in the physical need for exercise, helping and encouraging the patient to walk whenever and however many steps possible. The caregiver role may be filled by one or more persons, often impacted by the distance the hospital is from home.
Outpatient SCT procedures are significantly more demanding of caregivers. For the duration of treatment, the hospital will require the patient and caregiver(s) to be proximal to the hospital. Mayo, for example, requires patients to be within ten minutes of the hospital. Fortunately, there are many hotels, motels, inns, and homes for rent (HomeAway, VRBO) that are transplant-friendly and reasonably priced. It is 24/7 support, monitoring the patient’s key indicators, administering and monitoring meds, transporting the patient to/from the hospital daily, securing meds, shopping and preparing food, maintaining the household (e.g., laundry, sanitizing, etc.), and on and on. The list is extensive and exhaustive. Arranging for such intensive support can be a challenge. Some patients assemble a series of caregivers who rotate in/out for periods of time, others are able to secure one dedicated caregiver for the entire time, and in rare instances, the patient is able to have a team of caregivers for the duration.
Whichever caregiver structure is chosen, it is important to also consider self-care for the caregiver. Mini breaks can go a long way to help sustain their ability to meet the needs of the patient and the requirements set forth by the hospital.
From the Patient Perspective
For patients, it is all about getting through this treatment and hopefully arriving at a successful outcome. Time distills down to weeks, then days, and then when things are their most difficult, just getting through the next hour is the focus.
Having a good and capable caregiver(s) in place can help the patient focus only on themselves, knowing the caregiver will take care of everything else.
Side effects of the SCT can be multiple and vary from patient to patient. The list of effects can include fatigue, fever, diarrhea, nausea/vomiting, loss of appetite, mucositis, and hair loss. Fortunately, the healthcare team can be very helpful in mitigating these effects.
Exercise is important to ward off muscular atrophy and does improve recovery. Every step matters. Both Mayo and BU find patients do better and are home quicker if they spend less time in bed and more time moving around. In addition, patients tend to benefit from the required additional movement needed when living away from the hospital.
Emotionally, a SCT is tough. No way around that. But having distractions, whether provided by the caregiver, getting out of bed to exercise or being out and about via outpatient does contribute to an improved psyche. Having any sense of normalcy is welcome.
Cost differs greatly between inpatient and outpatient treatment, with outpatient coming in meaningfully less expensive. Anecdotal information has outpatient transplants at roughly 50% off the cost of inpatient transplants. Yet regardless of the approach, SCTs are extraordinarily expensive, and most likely patients need their insurance to sign off before treatment can begin. One of the considerations by insurance companies is which hospital the patient is proposing for treatment. During our personal experience, where we dealt with two national insurance companies, both informed us that having treatment at a Center of Excellence made a difference.
Finally, what is it really like? While situations vary widely from patient to patient, as may treatments and outcomes, hearing about a SCT straight from a patient who has been there is helpful. Having had an outpatient stem cell transplant in July 2017, hear Mackenzie’s perspective while fresh post-Mayo. Additionally, preparing for an outpatient SCT is more involved for the patient and caregiver; we have provided SCT and Post-Chemo Tips on the Resources page of our website which others may find helpful.
Closing Thoughts
There is strong evidence over many years and many transplants that patient outcomes are better when performed on an outpatient basis. There are, however, notable implications for the healthcare providers, patients and caregivers, depending on which approach is chosen. Inpatient, outpatient and hybrid approaches can provide successful outcomes, but knowing these differences in advance is helpful to the decision-making process.
——————————————
Special Thanks
Morie A Gertz, M.D., M.A.C.P.
Consultant | Division of Hematology | Roland Seidler Jr. Professor Department of Medicine | College of Medicine | Mayo Distinguished Clinician
Mayo Clinic
Vaishali Sanchorawala, M.D.
Professor of Medicine | Director, Autologous Stem Cell Transplant Program | Director, Amyloidosis Center
Boston Medical Center and Boston University School of Medicine
CRISPR/Cas9 – ATTR Clinical Trial Update
Per the National Institute of Health, “One of the most promising areas of research in recent years has been gene editing, including CRISPR/Cas9, for fixing misspellings in genes to treat or even cure many conditions.” In this piece we provide a clinical trial update for transthyretin (TTR) amyloidosis using this technology.
CRISPR FIXES GENES INSIDE THE BODY (3)
Per the National Institute of Health, “One of the most promising areas of research in recent years has been gene editing, including CRISPR/Cas9, for fixing misspellings in genes to treat or even cure many conditions.”
CRISPR is a highly precise gene-editing system that uses guide RNA molecules to direct a scissor-like Cas9 enzyme to just the right spot in the genome to cut out or correct disease-causing misspellings.
APPLYING THE CRISPR TECHNOLOGY (3)
Science highlights a small study reported in The New England Journal of Medicine by researchers at Intellia Therapeutics, Cambridge, MA, and Regeneron Pharmaceuticals, Tarrytown, NY, in which six people with hereditary transthyretin (TTR) amyloidosis, a condition in which TTR proteins build up and damage the heart and nerves, received an infusion of guide RNA and CRISPR RNA encased in tiny balls of fat.The goal was for the liver to take them up, allowing Cas9 to cut and disable the TTR gene. Four weeks later, blood levels of TTR had dropped by at least half.”
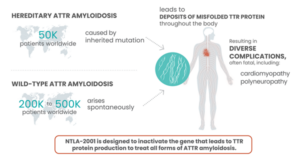
Facts about Transthyretin (ATTR) Amyloidosis. Source: https://ir.intelliatx.com/
CLINICAL TRIAL UPDATE — NTLA-2001 (1)
Intellia Therapeutics and Regeneron shared a press release recently announcing initial data from the cardiomyopathy arm of the ongoing Phase 1 trial of NTLA-2001, an investigational single-dose in vivo CRISPR-Cas9 therapy for the treatment of transthyretin (ATTR) amyloidosis.
According to that press release, the interim data include 12 adult patients with ATTR amyloidosis with cardiomyopathy (ATTR-CM) with New York Heart Association (NYHA) Class I – III heart failure. Single doses of 0.7 mg/kg and 1.0 mg/kg of NTLA-2001 were administered intravenously, and the change from baseline in serum transthyretin (TTR) protein concentration was measured for each patient. The data revealed that treatment with NTLA-2001 led to rapid and deep reductions of up to 94 % in serum TTR by day 28. In February 2022, the companies reported clinical data that revealed rapid, deep and sustained responses in a cohort of 15 patients with hereditary transthyretin (TTR) amyloidosis with polyneuropathy (ATTRv-PN).
ATTR is a rare, progressive disease, in which a protein known as TTR becomes misfolded and accumulates as plaques in tissues throughout the body. This causes serious complications that mainly involve the heart and nerves, and most patients die 2-15 years after disease onset. NTLA-2001 was the first in vivo CRISPR therapy to be administered to humans via the bloodstream. It is designed to treat ATTR by selectively reducing the levels of mutated TTR protein in the blood, through CRISPR-based inactivation of the TTRgene in liver cells.
Read more about the available clinical data for NTLA-2001 in a previous CMN clinical trial update here.
BACKGROUND
Back in May, 2021 we wrote about the breakthrough gene-editing technology CRISPR being applied to hereditary transthyretin amyloidosis (hATTR), worthy of a background read for those unfamiliar with this science or those looking for a refresher.
BLOG – CRISPR/Cas9 – Editing the Code of Life
Sources:
- CRISPR Medicine News: Special Update: News from the Gene-Editing Clinical Trials
- CRISPR Medicine News: CRISPR Therapy for Transthyretin Amyloidosis Results in Rapid and Prolonged Responses
- NIH Director’s Blog
- BLOG – CRISPR/Cas9 – Editing the Code of Life
FDA Approved AMVUTTRA for hATTR
Alnylam Announces FDA Approval of AMVUTTRA™ (vutrisiran), an RNAi Therapeutic for the Treatment of the Polyneuropathy of Hereditary Transthyretin-Mediated Amyloidosis in Adults
– First and Only FDA-approved Treatment Demonstrating Reversal in Neuropathy Impairment with Subcutaneous Administration Once Every Three Months
– AMVUTTRA Met Primary and All Secondary Endpoints, with Significant Improvement in Polyneuropathy, Quality of Life and Gait Speed Relative to External Placebo
– Company Expects to Launch in Early July, with Value-Based Agreements to Accelerate Access
The FDA approval is based on positive 9-month results from the HELIOS-A Phase 3 study, where AMVUTTRA significantly improved the signs and symptoms of polyneuropathy, with more than 50 percent of patients experiencing halting or reversal of their disease manifestations.
Following yesterday’s U.S. FDA approval, people in the U.S. prescribed AMVUTTRA (vutrisiran) and their families can now enroll in Alnylam Assist, our patient support services program, to receive help accessing this new therapy. https://bit.ly/3HjOg5Q

Seven Ways to Advocate for Your Health

If you have been diagnosed with a rare disease I’m sure that at some point you have met a nurse or doctor who has never heard of your condition. Some flat out say “what’s that?!” Some side eye you while they quickly google it on their phone. One nurse said to me once “Wow! If this was a teaching hospital everyone would want to come to see you.” Gee thanks. Way to make a girl feel special. Now I don’t blame them for this. Doctors and nurses have a very stressful and essential job and we would be lost without them. But they are human. And no one can be an expert in every condition or disease that exists. It’s impossible.
Which is why it’s a good idea to be an active member of your health care team and advocate for yourself to ensure your needs are being met.
Seven Ways to be an Active Member of Your Health Care Team
- Don’t take no for an answer! This is particularly important when you are searching for an elusive diagnosis. Don’t let them push you away or try to tell you that your symptoms are all in your head because they can’t figure you out. Trust your instincts. If you know something is wrong, go back again and again until they take you seriously. Get a second opinion. Don’t give up!
- Seek out experts. Once you have that diagnosis, do some research. Find the best specialists near you and ask to be referred to them. Ask your doctors, hit the internet, join a support group and reach out to others who have your disease. Ask around and find the experts! They are the ones you should put your trust in.
- Educate yourself. Don’t just go crazy with Google, as information in the public domain may be outdated. Ask your doctor for reputable sources of information. Join a support group, find others like you and learn from them. You can gain a wealth of knowledge from people who are living with your disease. Educating yourself is an important part of your healthcare.
- Ask questions! Why do I need this medication? What are the side effects? What do we do if I get those side effects? What happens if I don’t take this medication? Go through the risks and benefits of medications and treatments with your doctors. Don’t be afraid to ask questions. Your doctors want you to make informed decisions.
- Ask your expert before taking any medications or supplements. If you end up in the emergency room or a walk-in clinic, if time permits, run any suggestions for medications by your expert. Doctors who don’t have extensive knowledge of your rare disease may not know all of the potential complications and interactions. And don’t take any type of supplement or over the counter medication without first getting approval from your specialists. I love natural medicine, but it needs to be treated with the same respect and caution as any other medication. Natural does not equal safe.
- Organize your info and carry it with you. It can advocate for you if you’re not able to. I have a little folder which contains my official diagnosis report, a list of treatments I’ve had, a list of current medications, and the names and numbers of all my specialists. It comes along if I have to head to the emergency room.
- Speak up if you are suffering. I think sometimes we push through our suffering assuming that it’s just part of the process. But it may be as simple as adjusting the dosage or adding in another medication. So, if you are having a new or worsening symptom or a side effect from a medication, let your health care team know. There may be something they can do to help.
You are the most important member of your health care team. So, speak up when something is wrong, ask questions, learn as much as you can, and find the specialists that you can put your trust in. Self-advocacy can be a powerful force in your health care journey.
Lori Grover is a guest blogger for Mackenzie’s Mission. She was diagnosed with AL Amyloidosis in 2016 and writes to share experiences and lessons learned during her journey. More wonderful blogs by Lori can be found on her page Amyloid Assassin. When not writing, she is mostly a stay at home mom, florist, crafter, lover of books and food.
Fruit & Veggie Food Safety
Too lazy to wash your fruits and veggies? Here are a few statistics that may inspire you. The Centers for Disease Control and Prevention says about 48 million people are stricken with foodborne illness each year; 128,000 are hospitalized. Approximately 3,000 people die.
For patients with impaired or compromised immune systems, such as those going through chemo/stem cell transplant, practicing good food safety goes a long way.