
Amyloidosis can present in many types with the three most prevalent being light chain (AL) amyloidosis, hereditary variant transthyretin (ATTRv) amyloidosis, and wild type transthyretin (ATTRwt) amyloidosis. Being a rare disease, diagnosis can be particularly challenging, given that the general medical community is not well educated on the malady and symptoms are often associated with other more common ailments.
Successfully diagnosing the disease requires a two-step process before an appropriate treatment program can be determined and implemented for each patient.
- First, if amyloidosis is suspected, testing must be done to confirm the presence of amyloid.
- Second, once the presence of amyloid is confirmed, testing must then be done to identify and confirm the type of amyloidosis.
It is crucial that the second step, where the correct type of amyloidosis is identified, as the treatment regime can be different for each type. Here we share two different patient experiences which illustrate successful execution of the two-step diagnostic process.
Patient Case #1
The first case involved a 23-year old female. In 2017 she experienced an episode of coughing up blood, after which she looked in her throat with a flashlight and discovered a sizable lump. The patient met with a local ENT, who incorrectly diagnosed allergies, and prescribed over-the-counter medicine. With no improvement, she met with a second ENT. Testing was performed on the patient’s left oral pharynx utilizing a Congo red staining biopsy process which confirmed the presence of amyloid in the tissue. Additionally, mass spectrometry was performed which successfully differentiated the type of amyloidosis as being ALH (lambda light chain and delta heavy chain). Subsequently, she was referred to a hematologist who ordered a bone marrow biopsy and blood testing. The bone marrow biopsy summary notes read “….in conjunction with the concurrent finding of monoclonal lambda light chain restricted plasma cells in the marrow by flow cytometry, the findings are consistent with involvement of the marrow by a plasma cell neoplasm.”
Additionally, the blood testing confirmed elevated light chains as shown below.
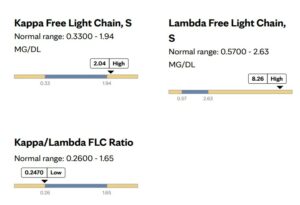
Patient Case #2
The second case involved a man in his mid-fifties. He began experiencing disease symptoms approximately 6-7 years prior to being diagnosed in early 2019. He initially experienced gradually progressing numbness in his feet, legs, hands and forearms, as well as bilateral carpal tunnel syndrome. Soon after, he began experiencing symptoms of lightheadedness and fainting. Additionally, he started experiencing progressive gastro-intestinal issues such as acid reflux, chronic coughing, and frequent bouts of constipation and diarrhea. By 2018, his physical condition was rapidly deteriorating, including a total weight loss of approximately 80 pounds. During this extended period of time he was seen by a variety of physicians including internal medicine, neurology, endocrinology, gastroenterology, oncology, and cardiology, none of who were successful in arriving at a conclusive diagnosis. His list of maladies included cardiomyopathy, peripheral neuropathy, autonomic neuropathy, bilateral carpal tunnel syndrome, and gastroparesis, all which are classic symptoms of amyloidosis.
Finally, in early 2019 his condition was successfully diagnosed by an amyloidosis specialist. An echocardiogram was performed as well as a cardiac MRI (utilizing a gadolinium tracer) to identify amyloid fibrils and related damage in the heart tissue. These tests confirmed the presence of amyloid. A free light chain serum test was performed which ruled out AL amyloidosis, and Transthyretin DNA sequencing was performed to differentiate between the hereditary variant and wild-type of ATTR, which identified the T80A (legacy T60A) variant of transthyretin (ATTRv) amyloidosis. The two tests were successful in identifying the type of amyloidosis. The associated testing results are show below.
Echocardiogram Summary Notes
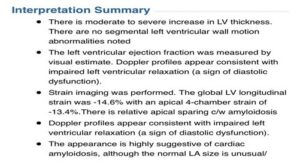
Associated Cardiac MRI Interpretation

DNA Sequencing Result

Once Diagnosed, Next is a Treatment Plan
Once the presence of amyloid is confirmed, and the type is identified, then it is time to treat the disease. In each of these patient cases the disease was diagnosed utilizing the two-step process to identify and confirm the type of amyloidosis. In both cases, successful treatment regimens were implemented which were effective in putting the disease into remission and/or halting disease progression.
Treatment options for amyloidosis have been vastly improved over the past several years. What was previously considered to be a foregone fatal disease can now be a manageable chronic disease. To ensure the best patient outcome, a timely diagnosis utilizing the two-step process, is essential.


