AN UPDATE ….. WOO HOO!!!
Well, the results of the preclinical studies were presented on June 26, 2021 and it is fantastic news for hereditary ATTR amyloidosis patients!!!
Preclinical studies showed durable knockout of TTR after a single dose. Serial assessments of safety during the first 28 days after infusion in patients revealed few adverse events, and those that did occur were mild in grade. Dose-dependent pharmacodynamic effects were observed. At day 28, the mean reduction from baseline in serum TTR protein concentration was 52% (range, 47 to 56) in the group that received a dose of 0.1 mg per kilogram and was 87% (range, 80 to 96) in the group that received a dose of 0.3 mg per kilogram.
CONCLUSIONS
In a small group of patients with hereditary ATTR amyloidosis with polyneuropathy, administration of NTLA-2001 was associated with only mild adverse events and led to decreases in serum TTR protein concentrations through targeted knockout of TTR. (Funded by Intellia Therapeutics and Regeneron Pharmaceuticals; ClinicalTrials.gov number, NCT04601051. opens in new tab.)
The New England Journal of Medicine
https://www.nejm.org/doi/full/10.1056/NEJMoa2107454
Our original blog post ….
The scientific world is abuzz … a Nobel Prize-winning technology called CRISPR/Cas9 can now edit our DNA. This programmable gene-editing technology, which is efficient, precise, and scalable, has inspired a gold rush of countless applications in medicine, agriculture and basic science. Early areas of focus include genetic diseases such as sickle cell and hereditary ATTR amyloidosis, offering new and exciting optimism.
Ground-Breaking Science in Gene Editing
“A genome is an organism’s complete set of DNA, including all of its genes. Each genome contains all of the information needed to build and maintain that organism. In humans, a copy of the entire genome – more than three billion DNA base pairs – is contained in all cells that have a nucleus.” – Intellia Therapeutics
CRISPR, short for Clustered Regularly Interspaced Short Palindromic Repeats, is a microbial ‘immune system’ that prokaryotes — bacteria and archaea — use to prevent infection by viruses called phages. At its core, the CRISPR system gives prokaryotes the ability to recognize precise genetic sequences that match a phage or other invaders and target these sequences for destruction using specialized enzymes.
Previous work had identified these enzymes, known as CRISPR-associated proteins (Cas), including one called Cas9. But scientist Emmanuelle Charpentier, working first at the University of Vienna and later at the Umeå Centre for Microbial Research in Sweden, identified another key component of the CRISPR system, an RNA molecule that is involved in recognizing phage sequences, in the bacterium Streptococcus pyogenes, which can cause disease in humans.
Charpentier reported the discovery in 2011 and that year struck up a collaboration with American biochemist Jennifer Doudna. In a landmark 2012 paper in Science, the duo isolated the components of the CRISPR–Cas9 system, adapted them to function in the test tube and showed that the system could be programmed to cut specific sites in isolated DNA – an incredibly precise set of DNA-editing genetic scissors. In 2020, Doudna and Charpentier won the 2020 Nobel Prize in Chemistry for their gene-editing technology.
“The ability to cut DNA where you want has revolutionized the life sciences,” said Pernilla Wittung Stafshede, a biophysical chemist and member of the Nobel chemistry committee, at the prize announcement. “The ‘genetic scissors’ were discovered just eight years ago, but have already benefitted humankind greatly.”
How Does CRISPR/Cas9 Work? (3)
This technology acts as an incredibly precise set of molecular scissors, providing instructions to cut an identified gene in a specific position in the nucleus of DNA. There are two primary components to the CRISPR/Cas9 genome editing system:
- The Cas9 protein, which initially recognizes the DNA and also acts like a pair of “molecular scissors” that precisely cleaves the targeted DNA sequence.
- The guide RNA, which guides the Cas9 scissors to the desired target DNA sequence and activates the scissors so they cut.
https://www.intelliatx.com/crisprcas9/how-crisprcas9-works/
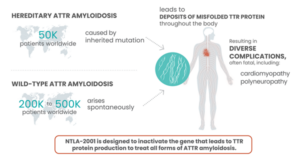
Background on Hereditary Transthyretin Amyloidosis (hATTR/ATTRv) (1)
Transthyretin amyloidosis is a slowly progressive condition characterized by the buildup of abnormal deposits of a protein called amyloid (amyloidosis) in the body’s organs and tissues. These protein deposits most frequently occur in the peripheral nervous system, which is made up of nerves connecting the brain and spinal cord to muscles and sensory cells that detect sensations such as touch, pain, heat, and sound. Protein deposits in these nerves result in a loss of sensation in the extremities (peripheral neuropathy). The autonomic nervous system, which controls involuntary body functions such as blood pressure, heart rate, and digestion, may also be affected by amyloidosis. In some cases, the brain and spinal cord (central nervous system) are affected. Other areas of amyloidosis include the heart, kidneys, eyes, and gastrointestinal tract. The age at which symptoms begin to develop varies widely among individuals with this condition, and is typically between ages 20 and 70.
There are three major forms of transthyretin amyloidosis, which are distinguished by their symptoms and the body systems they affect.
- The neuropathic form of transthyretin amyloidosis primarily affects the peripheral and autonomic nervous systems, resulting in peripheral neuropathy and difficulty controlling bodily functions.
- The leptomeningeal form of transthyretin amyloidosis primarily affects the central nervous system.
- The cardiac form of transthyretin amyloidosis affects the heart.
Mutations in the TTR gene causes the liver to product the TTR protein in a misfolded form. This misfolded protein can then build up in the body and lead to disease-causing nerve and other organ damage.
Clinical Trial Research (4)
According to CRISPRMedicineNews, one of the early clinical trials within gene editing is focused on hereditary transthyretin amyloidosis. In these trials, CRISPR-Cas is either used directly to treat the condition by editing an individual’s genome in vivo or indirectly through ex vivo engineering of a cell-based therapy. An update published November 17, 2020 discusses the clinical trial, which is now underway in the U.K.
CRISPR-Cas9 Trial For NTLA-2001 to Treat Hereditary Transthyretin Amyloidosis With Polyneuropathy
The second newly-added trial is sponsored by US-based Intellia Therapeutics and seeks to enroll 38 participants who are diagnosed with polyneuropathy (PN) due to transthyretin (TTR) amyloidosis (ATTR).
This open-label Phase 1 two-part trial comprises a dose escalation followed by a safety dose expansion study to evaluate the safety, tolerability, pharmacokinetics and pharmacodynamics of Intellia’s most advanced in vivo CRISPR-based therapy candidate, NTLA-2001.
ATTR is a hereditary progressive condition that is characterized by an accumulation of misfolded transthyretin (TTR) protein. The disease results from mutation(s) in the TTR gene, leading to mutant TRR protein that is unstable and easily forms aggregates that deposit as amyloid in various organs and tissues in the body. Organs or body parts most often affected include the nerves, heart, kidneys and eyes.
Life expectancy is typically 2-15 years from disease onset, and current treatment options include transplantation of affected organs and medications to slow progression of disease symptoms.
NTLA-2001 is the first investigative CRISPR-based therapy to be administered in vivo in humans. The new therapy comprises TTR-targeting gRNA and Cas9 mRNA, both of which are delivered in vivo via Intellia’s proprietary lipid nanoparticle technology. Pre-clinical studies support the notion that NTLA-2001 has potential as a one-time curative treatment. The first patient was dosed with NTLA-2001 last week and the study is expected to be completed in 2024.
Worldwide prevalence of spontaneous and hereditary transthyretin amyloidosis (ATTR). Source: Intellia Therapeutics. https://www.intelliatx.com/in-vivo-therapies/
Potential Game-Changer for Hereditary ATTR Amyloidosis
“Once we’ve assessed safety and established an optimal dose, we intend to rapidly initiate trials for the clinical manifestations of ATTR. NTLA-2001 may halt and reverse ATTR progression by producing a deeper, permanent TTR protein reduction for all patients – regardless of disease type – than the chronically administered treatments currently available.” said Intellia Therapeutics President and CEO, John Leonard, M.D.
Intellia’s proprietary CRISPR/Cas9 system could potentially address diseases with a single course of treatment because it permanently repairs the defective DNA. This represents a breakthrough improvement over current therapies, most of which require lifelong administration because they cannot correct underlying causes of the disease. However, this technology does not pass the genetic changes made to the patient to his or her offspring … the “fix” will not pass from generation to generation.
This is exciting news, giving new hope for families who have been ravaged by disease over generations.
———————————-
If you’d like to read more about Jennifer Doudna, here’s a book recently released by bestselling author Walter Isaacson, The Code Breaker.
Sources:
- https://crisprmedicinenews.com/clinical-trial/transthyretin-amyloidosis-attr-nct04601051/
- crisprmedicinenews.com
- https://www.intelliatx.com
- https://crisprmedicinenews.com/news/crispr-cas-clinical-trial-update/
- https://www.nature.com/articles/d41586-020-02765-9
- Doudna Lab, Berkeley, California
- CRISPR Therapeutics, Cambridge, Massachusetts
- Innovative Genomics Institute, Berkeley, California