
Clinical research is simply medical research involving people. Here we explore clinical trials and the basics of what you need to know, and how clinicians are working to improve racial and ethnic representation in amyloidosis clinical trials.
WHAT ARE CLINICAL TRIALS?
According to the National Institutes of Health (NIH), clinical trials are research studies performed on people that are aimed at evaluating a medical, surgical, or behavioral intervention. Clinical trials are the primary way that researchers find out if a new treatment, like a new drug or medical device (e.g., a pacemaker) is safe and effective in people. Often a clinical trial is used to learn if a new treatment is more effective and/or has less harmful side effects than the standard treatment. Other clinical trials test ways to find a disease early, sometimes before there are symptoms. Still, others test ways to prevent a health problem before it begins. A clinical trial may also look at how to make life better for people living with a life-threatening disease or a chronic health problem.
WHY CLINICAL TRIALS ARE IMPORTANT
Clinical trials permit researchers to test the safety and effectiveness of new therapies. They also allow for a rigorous evaluation through patient participation. Bottom line: it is only after the extensive evaluation and testing from a clinical trial that the FDA will approve the widespread use of any new therapy.
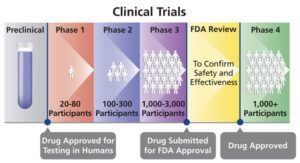
WHAT ARE THE PHASES OF CLINICAL TRIALS?
All clinical trials must be approved by the U.S. Food and Drug Administration (FDA) before they can begin. Prior to that decision, scientists perform laboratory tests and studies in animals to test a potential therapy’s safety and efficacy. Assuming favorable outcomes, the FDA then gives approval for a clinical trial involving humans.
Clinical trials are comprised of four phases to test a treatment, find appropriate dosages, and detect side effects. If following the completion of the first three phases, researchers find the drug or intervention to be safe and effective, the FDA approves it for clinical use and continues to monitor its effects. Overall, the duration of a clinical trial spans years.
WHY PEOPLE CHOOSE TO JOIN A CLINICAL TRIAL
There are many reasons why people choose to join a clinical trial. Some join a trial because the treatments they have tried for their health problem did not work. Others participate because there is no treatment for their health problem. Some studies are designed for, or include, people who are healthy but want to help find ways to prevent a disease that may be common in their family. By being part of a clinical trial, participants may access new treatments before they are widely available. Especially with a rare disease such as amyloidosis, clinical trials may offer a meaningful impact to a patient’s quality of life.
In addition, people may feel that participating in a clinical trial allows them to play a more active role in their own health care. Participants may receive more frequent health check-ups and closer monitoring through the clinical trial. Other people say they want to help researchers learn more about certain health problems. Whatever the motivation, when choosing to participate in a clinical trial, one becomes a partner in scientific discovery. This can also help future generations lead healthier lives. Major medical breakthroughs could not happen without the generosity of clinical trial participants—young and old.
POTENTIAL RISKS OF A CLINICAL TRIAL
There are no guarantees of success from a clinical trial, and there are risks. For starters, there may be serious side effects. Also, the therapy may not improve upon current treatment, or may not even work at all. Finally, as a clinical trial participant, you may be part of the control group, which means either standard treatment or no-treatment placebo. In other words, there are no assurances you would receive the new therapy.
FINDING A CLINICAL TRIAL
Thanks to the internet, folks can find lots of information regarding the wide array of open clinical trials. So much so that it may be overwhelming. Particularly with regards to amyloidosis, casting such a wide net may not be the most productive. Since finding an appropriate clinical trial is not easy for such a rare disease, here are a few excellent places to start.
- My Amyloidosis Pathfinder (MAP). Developed by the Amyloidosis Research Consortium (ARC), MAP helps patients discover and learn about amyloidosis-related clinical trials. After answering a short questionnaire, MAP matches patients to trials specific to their condition and ones for which they may be eligible.
- Amyloidosis Treatment Centers. The list of amyloidosis centers participating in clinical trials in the U.S. is growing. Below we list three active participating centers.
- ClinicalTrials.gov. This resource, provided by the U.S. National Library of Medicine, is a database of over 250,000 privately and publicly funded clinical studies conducted around the world (in all 50 states in the U.S. and 204 countries).
DECIDING WHO PARTICIPATES IN CLINICAL TRIALS
After signing the informed consent form, the clinical staff will screen the candidate against the clinical trial criteria. The screening may involve cognitive and physical tests. Inclusion criteria for a trial might include age, stage of disease, gender, genetic profile, family history, and whether or not the candidate has a study partner who can accompany them to future visits. Exclusion criteria might include factors such as specific health conditions or medications that could interfere with the treatment being tested. Generally, individuals can participate in only one trial or study at a time. Different trials have different criteria, so being excluded from one trial does not necessarily mean exclusion from another.
In the following video, Dr. Frederick Ruberg, cardiologist at the Boston University Amyloidosis Center, discusses the diversity of racial and ethnic occurrences of amyloidosis, and why this should parallel patient representation in clinical trials. He illustrates the persistent disparities observed by race and ethnicity and how un-recognized ATTR amyloidosis, for example, could be contributing to such differences. Adding more evidence, he shares how published clinical trials of ATTR-CM agents are insufficiently diverse. He summarizes possible solutions for improving future clinical trial participation.
Clinical trials need numbers and diversity of patients that reflect the patient community. Many volunteers must be screened to find enough people for a study, and with rare diseases such as amyloidosis, important trials are often significantly delayed due to a lack of participants. This can seriously slow down the rate at which new drugs are discovered, tested, and made available to patients.
CONCLUSION
Not all clinical trials have successful outcomes. However, every disease drug and therapy treatment prescribed today is the result of clinical research. Clinical trials are absolutely necessary to determine that a treatment is safe and that it has a real positive effect on a particular disease, better than that observed by a placebo or the current standard of care. In addition, ensuring participant diversification adequately reflects the patient population is an important consideration when recruiting for clinical trials. Clinical trials are how we will learn more about amyloidosis, discover new drugs and treatment therapies, and find a cure.
Additional information regarding clinical trials, including where to find clinical trials and participant protection and safety, can be found in Clinical Trials 101.
==========================================================================
SOURCES
Amyloidosis Research Consortium
Boston University / Boston Medical Center, Amyloidosis Center
The Clinical Study Center
Mayo Clinic
National Institute on Aging
National Institutes of Health
Stanford University Amyloid Center
U.S. National Library of Medicine
