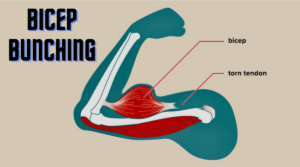
Often called “Popeye Deformity,” bicep bunching is visible when the patient flexes their arm, giving the appearance of Popeye-like arms. While it is the result of a torn tendon, it can be a leading indicator of more serious issues.
WHAT IS IT?
When the bicep tendon is ruptured, patients develop a bunching of the biceps upon flexion of the arm against gentle resistance. Tendon ruptures occur largely in the dominant arm of each patient, with one-quarter of patients developing ruptures in both arms. Interestingly, of those who had a rupture, 37.8% didn’t know it.
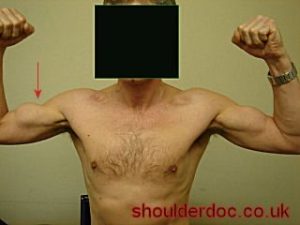
Below watch a video from The Lancet showing what bicep bunching looks like.
WHAT DOES IT POTENTIALLY INDICATE?
Two things.
1. Bicep bunching may be a marker for ATTRwt. According to MedPage Today, spontaneous ruptures of the distal biceps tendon may be a marker of wild-type transthyretin (TTR) cardiac amyloidosis, a single-center study found. The presentation of a tendon rupture, an easily elicited diagnostic sign, in a patient with HFpEF should raise suspicion for wild-type TTR cardiac amyloidosis.
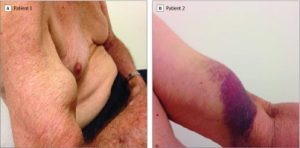
The picture below (Source: JAMA September 12, 2017 Volume 318, Number 10) offers examples of ruptured biceps tendon in two patients with biopsy-proven ATTRwt Cardiac Amyloidosis. ATTRwt indicates wild-type transthyretin amyloidosis. Patient 1 with prior rupture of the biceps tendon and bunching of the biceps with flexion. Patient 2 with acute rupture of the biceps tendon in the left arm; the tendon rupture occurred with trivial trauma, five years after Cardiac Amyloidosis diagnosis.
2. ATTRwt may contribute to heart failure. Wild-type transthyretin amyloidosis (ATTRwt) is increasingly recognized as an important cause of heart failure with preserved ejection fraction (HFpEF).
WHY IS IT IMPORTANT?
Bicep bunching may be a marker of wild-type transthyretin (TTR) cardiac amyloidosis, potentially giving physicians an easy way to determine the underlying cause of heart failure with preserved ejection fraction (HFpEF) in some patients. Those who were aware, reported that the distal biceps tendon ruptured approximately five years prior to heart failure diagnosis, thus perhaps offering a leading insight.
In addition, early diagnosis of wild-type TTR cardiac amyloidosis (ATTRwt) is important because treatments are now available to slow, if not halt, disease progression. Unfortunately, the diagnosis of ATTRwt is often not considered in bicep bunching cases due to the perceived rarity of the disease.
“The clinical importance [of this study] is that the detection of a ruptured distal biceps tendon may be a clue for the diagnosis of wild-type TTR amyloidosis as the cause for heart failure. This diagnosis is often overlooked in clinical practice, so this relatively simple evaluation could increase detection of the disease,” said Stuart Katz, MD, of NYU Langone Health. “Enhanced detection could lead to better treatment.”
EXPERT INSIGHTS VIDEO ON MUSCULOSKELETAL MANIFESTATIONS
Dr. Shari Liberman, a hand and upper extremities surgeon from Houston Methodist Orthopedics & Sports Medicine, discussed six orthopedic manifestations and their pathology as it relates to systemic amyloidosis. Published studies, coupled with her experience, has led to a belief that these manifestations can offer important evidence of amyloidosis. She concludes with thoughts regarding an orthopedic differential and biopsy considerations for each of these manifestations.